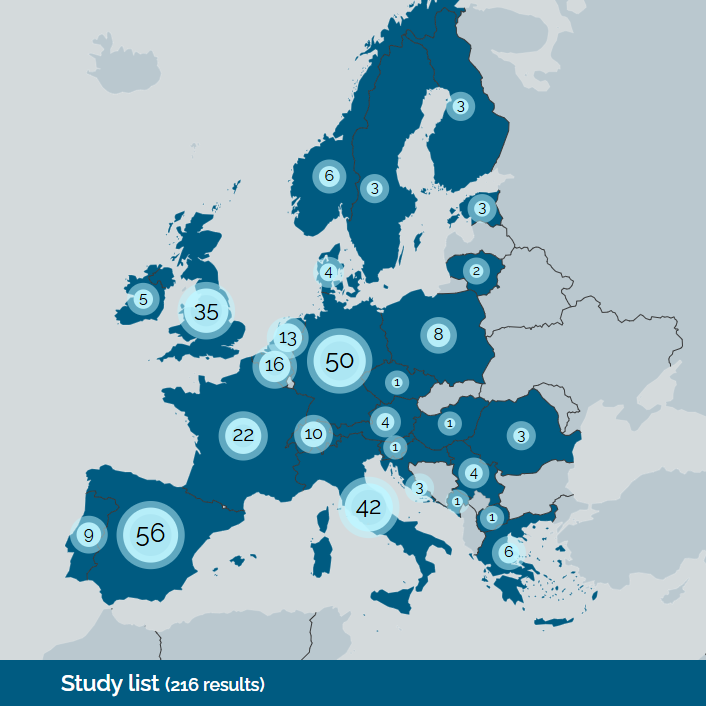
On Tuesday 16 December, the Cohort Coordination Board (CCB) celebrated its 40th meeting since it’s conception back in April 2022. The CCB, coordinated by the ID-CARE team at the University of Verona, is a forum that brings together cohort-based ID research projects across Europe alongside stakeholder representatives, aiming to facilitate partnerships, find solutions to common challenges