The Central Data Repository (CDR) is a publicly accessible online database of clinical research studies targeting infectious diseases with epidemic or pandemic potential in Europe. The CDR will be updated regularly to include new studies according to new global threats and European epidemiological scenarios.
Why was the CDR developed?
The CDR was developed as part of the EU-funded VERDI (GA-101045989) and CoMeCT (GA-101136531) projects. It aims to streamline data sharing, foster collaboration, and prevent duplication in clinical research efforts targeting infectious diseases with epidemic or pandemic potential. The CDR provides key stakeholders and researchers with an overview of adaptive platform trials (APT), randomised clinical trials (RCT), and cohort studies (CS) ongoing or conducted from January 2022, focusing on emerging threats or public health emergencies of international concern. This facilitates rapid identification and activation of suitable infrastructures and clinical sites, enhancing coordination of European research, and linking relevant initiatives and networks. By preventing redundant research efforts, the CDR accelerates scientific progress and optimises resource allocation, ultimately improving public health response.
What clinical research studies are included in the CDR?
The CDR includes CSs, RCTs and APTs, carried out in Europe that focus on infectious diseases, particularly those that may be considered emerging threats. Currently, the CDR includes studies that analyse acute COVID-19 (treatment; RCT mapping ongoing), post-COVID-19 syndrome (natural history, treatment), mpox, avian influenza A(H5N1), respiratory syncitial virus, chikungunya virus disease, dengue, zika, west nile virus disease, chlamydia trachomatis infection and neisseria gonorrhoeae infection.
After filtering or searching for relevant conditions or keywords, the user receives a list of clinical studies and/or APT. Each study is named after the study or cohort acronym, or if unavailable, the principal investigator of the study.
What information can be found in the CDR?
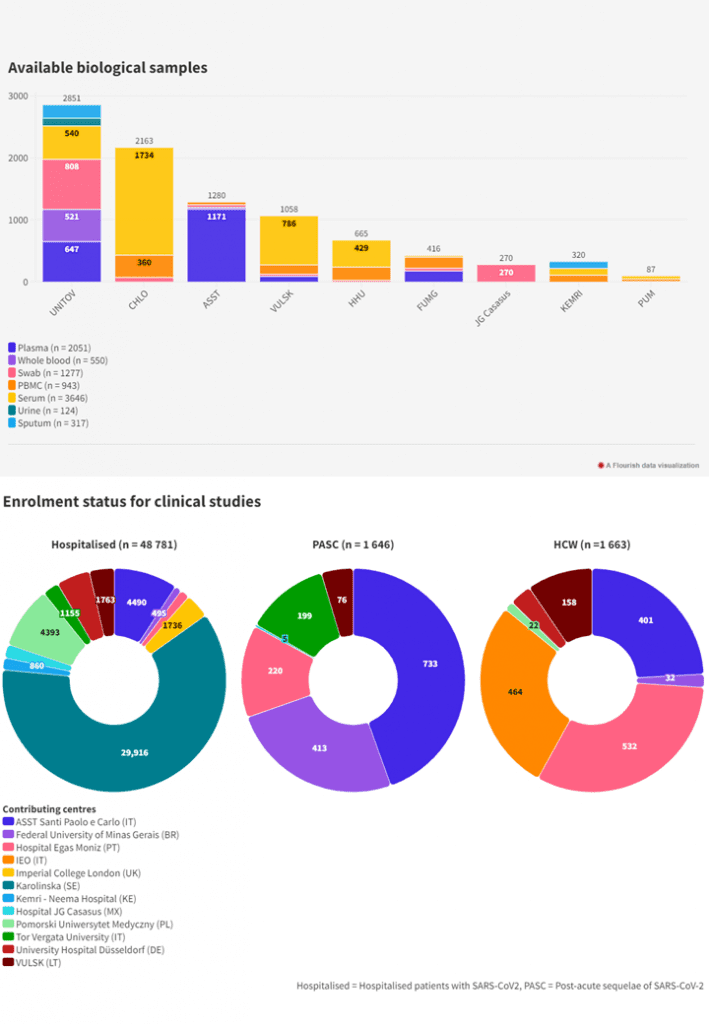
The CDR consists of three parts: interactive filters, a map of Europe showing the number of studies per country, and a browsable list of the studies. The map and the study list show the studies according to the selected filters. Each study in the CDR is detailed in a Study Identity Card, which includes the study’s name, design, number of participants, enrolment dates, location, population, setting, pathogen, disease, and biobanked samples. Whenever available, a link to the project website, study registration log and/or publication link is provided. The Cohort Coordination Board can be also contacted to support the linking of the researchers and initiatives.
How is information collected for the CDR?
Studies are identified across several publicly available sources (PubMed, Scopus, ECRIN, CTIS, ClinicalTrials.gov), and through direct contact with the Cohort Coordination Board members, the VERDI and COMECT scientific networks, and other relevant researchers. The screening of the studies is carried out applying an artificial intelligence (AI)-supported tool, supervised by infectious diseases specialists. Study metadata is provided by principal investigators and/or extracted from publicly available sources, employing a number of automated and semi-automated methods. The studies are all assigned applicable highly curated descriptors, enabling rapid and accurate browsing.
Future developments
- Development of a second module reporting data dictionaries of included studies
- Addition of a syndromic approach to enlarge the mapping activities and support rapid identification of clinical sites able to run new clinical studies (as for viral respiratory infections and H5N1); first module will be influenza-like illnesses
- Catalogue and align the variables from the CDR studies, enabling seamless identification of compatible datasets.
Terms and conditions
The CDR is a platform constructed to provide an overview of the studies running in Europe. While the data is provided in good faith and to the best of our knowledge, neither UNIVR nor any VERDI/COMECT partners make any warranty, express or implied, including warranties of fitness for a particular purpose, or assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information provided (either isolated or in the aggregate), or represents that its use would not infringe privately owned rights.
The data is intended for Educational and Research purposes only. The term Educational Purposes means* (i) in the case of a Qualified Educational Institution, Faculty or Other Authorized Educational Licensees, purposes directly related to learning, teaching, and training, that are part of the instructional functions performed by a Qualified Educational Institution or Other Authorized Educational Licensee and (ii) in the case of an individual, purposes related to learning and training. “Educational Purposes” does not include research, commercial, professional, or any other for-profit purposes.
Get in touch
We are actively looking to collaborate with other initiatives working in this area, and we welcome you to contact us if you are interested in collaborating, including or amending data onto the CDR.
Legend
Fragile population
Individuals who are more vulnerable to poor health outcomes due to factors like chronic illness, weakened immune systems, disabilities or socioeconomic disadvantages
General population
Individuals who do not belong to a defined subgroup with specific characteristics, representing the broader population
Healthcare workers
A professional providing medical care, support, or public health services in various healthcare settings
Immunocompromised host
An individual with an impaired immune system due to conditions such as HIV, cancer, organ transplantation, or immunosuppressive therapy
Men who have sex with men
Men who have sex with men are men who engage in sexual activity with other men, regardless of their sexual orientation or sexual identity
People at high risk of STIs
Individuals with an increased likelihood of acquiring sexually transmitted infections (STIs) due to behavioral, biological, or social factors
People living with HIV
Individuals who have been diagnosed with human immunodeficiency virus (HIV) and may be receiving antiretroviral therapy to manage the condition
PrEP users
Individuals who take pre-exposure prophylaxis (PrEP), a medication regimen to prevent HIV infection
Community
A research environment where studies are conducted outside of clinical or laboratory settings, focusing on real-world populations
Hospital
Encompassing all units and departments within a hospital setting
Infectious diseases unit
Hospital ward specialised in infectious diseases
Intensive care unit
Hospital intensive care unit (ICU) ward
Long-term care facility
A healthcare institution that provides continuous medical and personal care
Non-hospital health centre
Healthcare facilities outside hospitals, such as long term care facilities, community health centers, and vaccination centers
Outpatient clinic
A healthcare facility where patients receive medical care, consultations, or treatments without being admitted to a hospital
Primary care
The first level of healthcare, including general medical services, such as family medicine
STD/STI clinic
A specialized healthcare facility that provides testing, diagnosis, treatment, and counseling for sexually transmitted diseases (STDs) and infections (STIs)
Telemedicine
The remote delivery of healthcare services using digital technology
Vaccination centre
A healthcare facility or designated location where vaccines are administered to individuals. It may operate in hospitals, clinics, pharmacies, or temporary sites for mass immunization campaigns
Antineoplastic agent
Drugs used to inhibit or stop the growth of cancer cells. Examples include chemotherapy, targeted therapy, and immunotherapy agents
Antivirals
A class of medications used to treat viral infections
Dietary
A type of lifestyle intervention that aims to change a patient's diet
Immunomodulator
Drugs used to modify the immune system's response, either by enhancing or suppressing it
Lifestyle
Interventions striving to intoduce a permanent change in the patient's lifestyle. Examples include changes to excercise routine, diet or sleep
Non pharmacological intervention
All types of intervention that are not pharmacological, not including diagnostic
Pharmacological intervention
This refers to the drug, therapy, or factor being studied in a clinical trial or observational study. Examples include antiviral agents monoclonal antibodies, corticosteroids, vaccines and other pharmacological agents
Probiotic
Pharmacological compounds aimed at the modification of the gut microbiome
Rehabilitation
All non-pharmacological interventions based on physical or cognitive rehabilitation of the patient
Statins
Class of medications that lower cholesterol levels